Chemical Properties of Carboxylic Acids
Chemical Properties of Carboxylic Acids: Overview
In this topic, we will learn the chemical properties of carboxylic acids with the help of examples.
Important Questions on Chemical Properties of Carboxylic Acids
The correct increasing order of acidic strength of the following compounds is
benzoic acid
nitro benzoic acid
, dinitrobenzoic acid
methoxybenzoic acid
Identify A to D in the following sequences of operations:
The reagents that can be used to distinguish between the following pairs of compounds would be:
(i) Propanal and Propanone
(ii) Phenol and Benzoic acid
Among the following acids which has the lowest value?
A liquid was mixed with ethanol and a drop of concentrated H2SO4 was added. A compound with a fruity smell was formed. The liquid was:
A liquid was mixed with ethanol and a drop of concentrated H2SO4 was added. A compound with a fruity smell was formed. The liquid was:
Which amongst the following compounds has the highest acid strength?
Given below are two statements, one is labelled as Assertion and the other is labelled as Reason .
Assertion : A solution of the product obtained by heating a mole of glycine with a mole of chlorine in presence of red phosphorous generates chiral carbon atom.
Reason : A molecule with chiral carbons is always optically active.
In the light of above statements, chose the correct answer from the options given below:
Match List-I with .
|
List-I Name of reaction |
List-II Reagent used |
||
| Hell-Volhard Zelinsky reaction | |||
| Iodoform reaction | |||
| Etard reaction | |||
| Gatterman-Koch reaction | |||
Choose the correct answer from the options given below:
The major product formed in the following reaction is
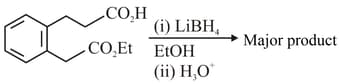
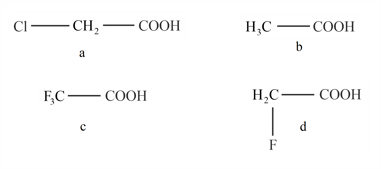
Compare the acid strength of:
Find the correct order of acidity of the following compounds :
Match the following
| Name of the Reaction | Reagents |
| (a) Etard reaction | (p) |
| (b) Iodoform | (q) |
| (c) Gattermann | (r) |
| (d) HVZ | (s) |
Identify the products.
Which of the following acids has least acidic strength ?
Ethanoic acid undergoes Hell-Volhard Zelinsky reaction but Methanoic acid does not, because of
The reagent that can reduce the carboxylic acid group to the corresponding alcohol is
The major product of the following reaction is
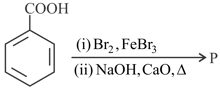
The major product of the following reactions is

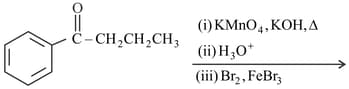
An aryl carboxylic acid on treatment with sodium hydrogen carbonate liberates a gaseous molecule. Identify the gas molecule liberated.
